
Dr. Gilles Guichard
Directeur de recherche (DR2), CNRS
Cette adresse email est protégée contre les robots des spammeurs, vous devez activer Javascript pour la voir.
Website
Tel: +33(0)540003020
Bio
Gilles Guichard graduated in chemistry from the Ecole Nationale Supérieure de Chimie in Toulouse (1991) and University of Montpellier (1992) in France. He received his PhD from the University Louis Pasteur in Strasbourg (1996), working on immune recognition of pseudopeptides and synthetic vaccines. Following post-doctoral research with Prof. Dieter Seebach at the ETH in Zürich (1997) in the field of β-peptide foldamers, he joined the Institut de Biologie Moléculaire et Cellulaire (IBMC) in Strasbourg as a CNRS Chargé de Recherche (1998). Since 2006, he has been a CNRS Research Director. In 2009, he moved as a new group leader to the Institut Européen de Chimie et Biologie (IECB) in Bordeaux. His current research focuses on biomimetic chemistry of peptides, foldamer chemistry, self-assembly and biomolecular recognition.
Keywords / Expertise / Techniques
peptide and peptidomimetic chemistry, foldamers, helices, oligoureas, solid-phase and combinatorial techniques, multimeric architectures, self-assembly, conformational studies, molecular recognition, antibacterial agents, death receptors, protein-protein interactions.
Summary
The ability of the polypeptide chain to fold correctly into well-ordered tertiary structures that can ultimately assemble into defined quaternary arrrangments is a major determinant of protein function. Multiple approaches, at the interface between biology, synthetic organic and polymer chemistries are currently being explored to elaborate synthetic systems with protein-like structures and functions. By using peptidomimetic chemistry, the general aims of our research are (i) to understand how to program molecules with the necessary information for self-ordering into complex and functional architectures, (ii) to create folded systems mimicking protein secondary
Activity report
Our research program focuses on Peptidomimetic and Foldamer Chemistry and more specifically on aliphatic urea oligomers which adopt well defined and stable helical secondary structures. Over the last five years, the group has thoroughly explored the requirements for secondary structure formation, expanded the monomer repertoire and improved synthesis by generalizing solid-phase methods. The development of functional folded architectures has now become a major objective. Our early work in this direction was mainly dedicated to the design of cationic amphiphilic helices with antibacterial activities and was recently extended to sequences that interact with plasmid DNA (Highlight #1). The finding that oligourea foldamers can be interfaced with peptide helices is another feature of particular significance in the context of peptide and protein mimicry (Highlight #2). Finally, we have started to explore the possibility to design foldamer units for the precise construction of nanometer scale assemblies mimicking protein quaternary structures (Highlight#3).
2015 Highlights :
Highlight #1 : A Cell-Penetrating Foldamer for Intracellular Delivery of DNA
Peptide mimics based on artificial backbones may combine useful properties such as diminished susceptibility to degradation by proteases, high control over secondary structures, and a propensity to form assemblies in aqueous solution. Despite significant advances in foldamer chemistry, tailored delivery systems based on foldamer architectures, are curiously rare among non-viral technologies for transporting nucleic acids into cells. In this work, we sought to develop a urea-based cell-penetrating foldamer system for gene transfection that would enable endosomal escape and release of DNA into the cytoplasm. We prepared pH-responsive and bioreducible cell-penetrating foldamers (CPF) through covalent dimerization of a short (8-mer) amphipathic oligourea sequence bearing histidine-type units. This CPF was found to assemble with pDNA and to mediate efficient delivery of nucleic acids into the cell (Angew. Chem. Int. Ed. 2015).
Highlight #2 : Chimeric α-Peptide/Oligourea Helices
We have obtained very promising results trying to interface urea-based foldamers with α-peptides (Angew Chem Int Ed 2015 & C R Chimie 2016). The rationale was that the oligourea helix shares a number of features with the α-helix such as helix polarity and pitch. In this work (see Figure 1), we have investigated the ability of aliphatic oligoureas fused to short peptide segments to nucleate α-helical structures. We found that the resulting chimeras were fully helical in the solid state as well as in polar solvents, with as few as 2 (or 3) urea units sufficient to propagate an α-helical conformation in the fused peptide segment. The remarkable compatibility of α-peptide and oligourea backbones, along with the simplicity of the technology may suggest a general approach for the mimicry of peptide and protein helices for various applications.
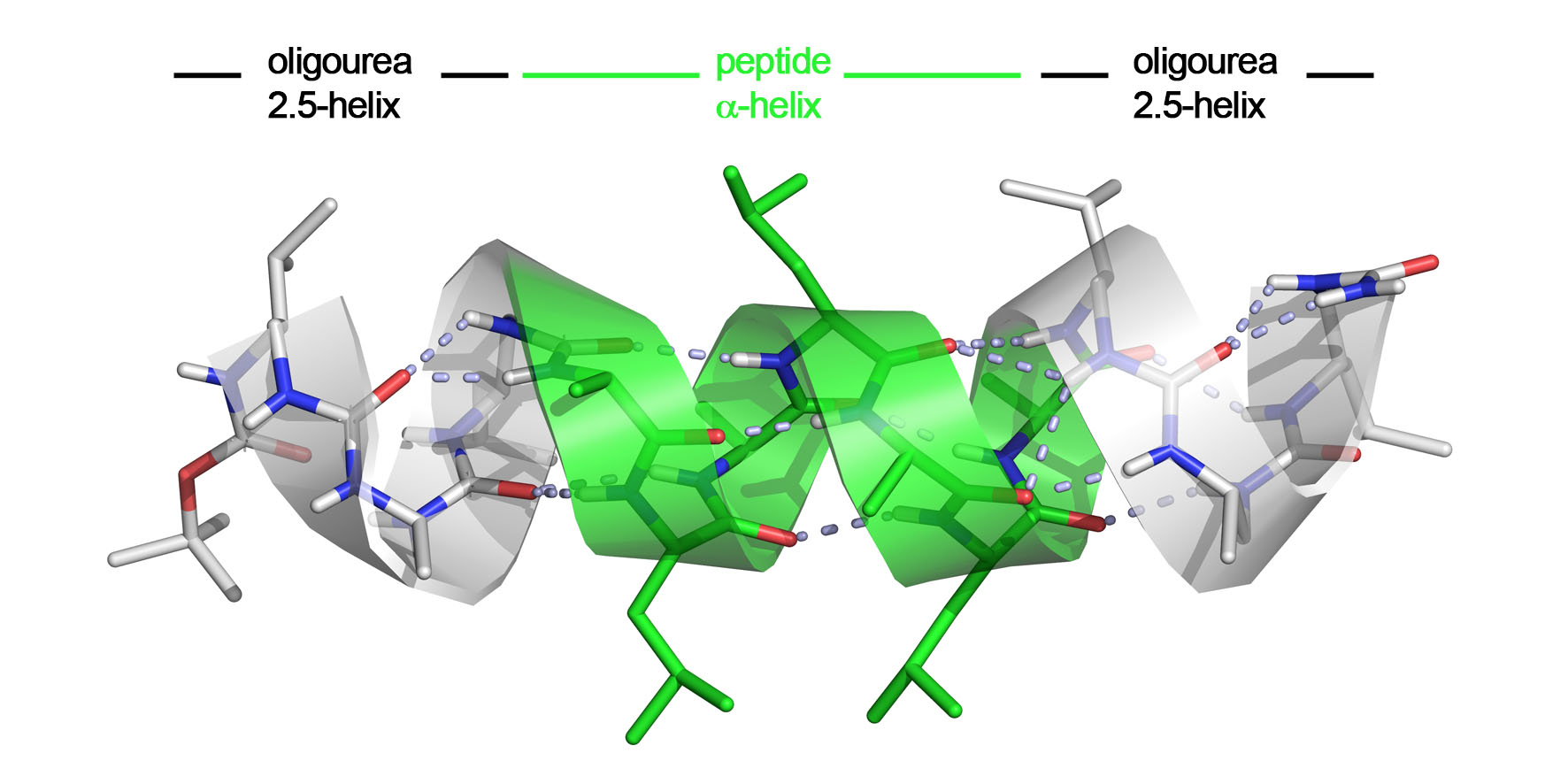
Fig. 1. Crystal structure of a α-helix (green) stabilized by two short adjacent accessory foldamers (grey).
Highlight #3 : Self-assembly of Amphiphilic Non-peptide Helical Foldamers
Taking inspiration from protein tertiary and quaternary structures, we have successfully transposed folding and assembly processes to artificial (non-peptide) molecular chains to create nanostructures with unusual shapes (e.g. bundles or nanotubular assemblies) in aqueous conditions (Nature Chem, 2015 & Chem Commun 2016). Four high-resolution crystal structures of unique foldamer quaternary arrangements have been described, supported by high-field NMR, electron microscopy and additional solution studies (Figure 2). The sequences of these oligomers were designed to contain a certain proportion of residues with polar and nonpolar side chains which depending on their arrangement at the surface of the helix determine how the helices self-assemble in water. Similar to soluble proteins, the hydrophobic effect (which leads to the burial of nonpolar side chains) is predominant. Due to (i) the simplicity of the design hypothesis, (ii) the small size of helical components and (iii) the tunability of the reported assemblies, this approach could be implemented for the creation of structures with a wider range of topologies, functional properties and, consequently, applications.
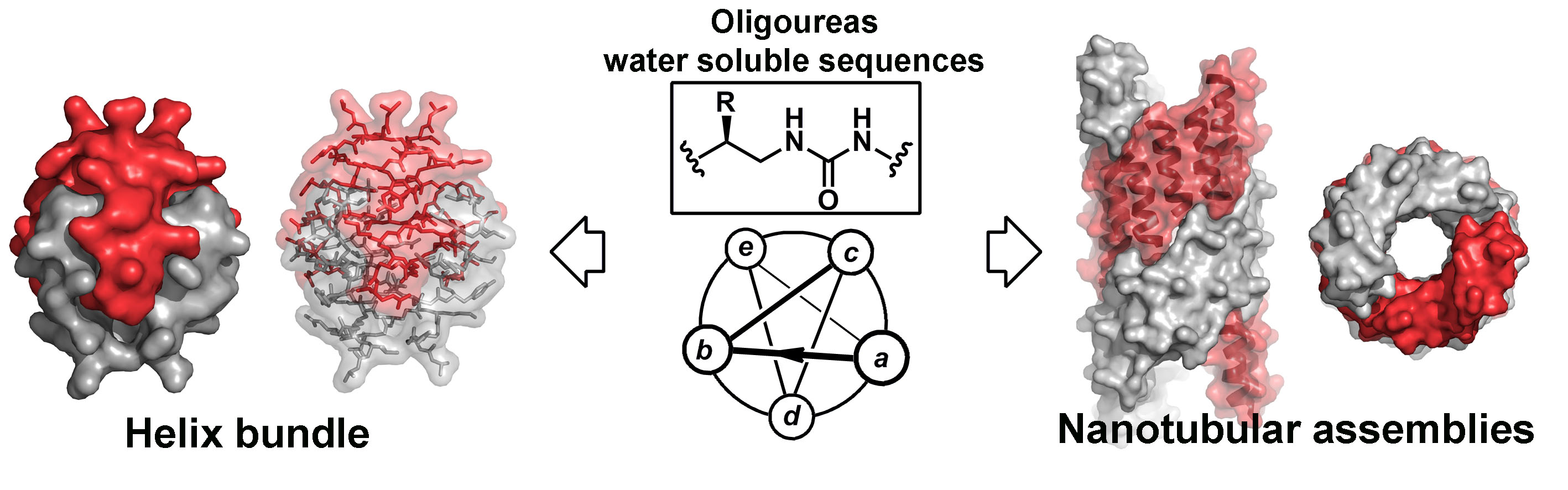
Fig. 2. Assemblies obtained in aqueous conditions from amphiphilic oligourea sequences.
Selected publications
- Collie, G. W.; Pulka-Ziach, K.; Guichard, G. In situ iodination and X-ray crystal structure of a foldamer helix bundle. Chem. Commun. 2016, 52, 1202-1205.
- Collie, G. W.; Pulka-Ziach, K.; Lombardo, C. M.; Fremaux, J.; Rosu, F.; Decossas, M.; Mauran, L.; Lambert, O.; Gabelica, V.; Mackereth, C. D.; Guichard, G. Shaping quaternary assemblies of water-soluble non-peptide helical foldamers by sequence manipulation. Nat Chem 2015, 7, 871-878.
- Douat, C.; Aisenbrey, C.; Antunes, S.; Decossas, M.; Lambert, O.; Bechinger, B.; Kichler, A.; Guichard, G. A Cell-Penetrating Foldamer with a Bioreducible Linkage for Intracellular Delivery of DNA. Angew. Chem. Int. Ed. 2015, 54, 11133-11137.
- Fremaux, J.; Mauran, L.; Pulka-Ziach, K.; Kauffmann, B.; Odaert, B.; Guichard, G. α-Peptide–Oligourea Chimeras: Stabilization of Short α-Helices by Non-Peptide Helical Foldamers. Angew. Chem. Int. Ed. 2015, 54, 9816-9820.
- Seefeldt, A. C.; Nguyen, F.; Antunes, S.; Perebaskine, N.; Graf, M.; Arenz, S.; Inampudi, K. K.; Douat, C.; Guichard, G.; Wilson, D. N.; Innis, C. A. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat Struct Mol Biol 2015, 22, 470-475.
- Nelli, Y. R.; Antunes, S.; SalaĂĽn, A.; Thinon, E.; Massip, S.; Kauffmann, B.; Douat, C.; Guichard, G. Isosteric Substitutions of Urea to Thiourea and Selenourea in Aliphatic Oligourea Foldamers: Site-Specific Perturbation of the Helix Geometry. Chem. Eur. J. 2015, 21, 2870-2880.
- Pulka-Ziach, K.; Pavet, V.; Chekkat, N.; Estieu-Gionnet, K.; Rohac, R.; Lechner, M.-C.; Smulski, C. R.; Zeder-Lutz, G.; Altschuh, D.; Gronemeyer, H.; Fournel, S.; Odaert, B.; Guichard, G. Thioether Analogues of Disulfide-Bridged Cyclic Peptides Targeting Death Receptor 5: Conformational Analysis, Dimerisation and Consequences for Receptor Activation. ChemBioChem 2015, 16, 293-301.
- Wechsel, R.; Maury, J.; Fremaux, J.; France, S. P.; Guichard, G.; Clayden, J. Inducing achiral aliphatic oligoureas to fold into helical conformations. Chem. Commun. 2014, 50, 15006-15009.
- Wolff, P.; Amal, I.; Oliéric, V.; Chaloin, O.; Gygli, G.; Ennifar, E.; Lorber, B.; Guichard, G.; Wagner, J.; Dejaegere, A.; Burnouf, D. Y. Differential Modes of Peptide Binding onto Replicative Sliding Clamps from Various Bacterial Origins. J. Med. Chem. 2014, 57, 7565-7576.
Research team
The team is part of the unit “Chimie et Biologie des Membranes et Nanoobjets” (CBMN), CNRS – Université de Bordeaux - Bordeaux INP (UMR 5248)
|