
Pr. LĂ©on Ghosez
Emeritus Professor UCL, Visiting scientist IECB
Website
Cette adresse email est protégée contre les robots des spammeurs, vous devez activer Javascript pour la voir.
Tel: +33(0)540002217
Bio
Léon Ghosez was born in Aalst, Belgium, in 1934. He studied at the University of Louvain where he got a PhD in 1958 under the supervision of Prof. G. Smets. He then spent 2 years as postdoctoral researcher at Harvard University (Prof. R.B. Woodward). He also collaborated for a few months with Prof. R. Huisgen at the University of Munich. He got his “Habilitation” in 1969 at the age of 32 and became Professor” at the University of Louvain. During his career in Louvain (1963-1999) he supervised the research of 125 PhD students and 135 postdoctoral associates. He also held appointments at the University of Liège (1969-1999) and the Ecole Polytechnique in Palaiseau (1993-1999). He took an active part in the creation of IECB where he established a research group in 1998 and from 2000 till the end of 2009, he shared the directorship of IECB with Dr. J.J. Toulmé. Presently he is a visting scientist at IECB and Prof. Emeritus at UCL. Léon Ghosez is an Emeritus Member of the Royal Academy of Sciences, Literature & Fine Arts of Belgium. He recently received the Medal of the French Chemical Society.
Keywords / Expertise / Techniques
total synthesis, diversity, synthetic methods, electrophilic catalysis, convertases ,kinases, apoptosis, tubulin, serine proteases, Blc-2, synthesis, medicinal chemistry, structural studies by spectroscopy.
Summary
Project 1: Small natural molecules have been shaped and optimized by evolution and are therefore perfectly tailored to interact with natural macromolecules and induce a biological response. Our first research project consists in designing and producing privileged scaffolds by short sequences of reactions. These should be readily transformed into a wide diversity of natural product analogs of therapeutic interest. This provides an entry into the drug discovery process at a much more advanced stage that does the screening of standard diversity libraries.
Project 2: Our goal is to develop new ionic solvents and silicon-derived Lewis superacids which are both derived from strong Brönsted acids of rather low molecular weight. These are used as functionally tolerant though highly electrophilic solvents or catalysts for reactions highly functionalized molecules. These solvents and catalysts are free of toxic metals and leave little waste allowing for sustainable processes
Activity report
1. Synthesis and evaluation of new ligands of the glucocorticoid receptor
Spirocyclic glucocorticoids were designed, synthesized and evaluated for their activity toward hGRs and IL1/IL6 receptors. A diastereoselective approach was conducted in order to evaluate the biological activity of specific diastereoisomers. The synthetic sequence, without details, is shown in scheme 1. The replacement of fused- by spiranic rings give more conformational freedom to ring C. Thus both diastereomeric alcohols were shown to have an axial hydroxyl group in the most stable conformation. The homologues in 5-, 6- and 7-series have different conformations with huge impact on their binding properties. Our synthetic studies were supported by several single crystal X-rays analysis and DFT calculations performed by Dr. F. Robert of ISM, Univ. Bordeaux. This project led to interesting biological findings. Compounds with nanomolar ranges of activities were obtained and, more interestingly, we found dissociation of the activity profile (hGRs vsIL1/IL6 receptors) for some compounds. This study also led to an unprecedented application of Burgess reagent (Scheme 2) which led an interesting expansion of ring B.
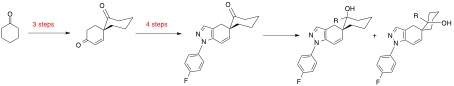
Scheme 1. A short synthesis of new spirocyclic modulators of the glucocorticoid receptor
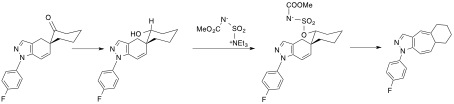
Scheme 2. An unprecedented application of Burgess reagent
2. A versatile and productive Diels-Alder route to NPs-inspired privileged scaffolds
The group has pursued its effort towards the synthesis of privileged scaffolds designed after natural products templates. This chemistry-driven approach to drug design relies upon the availability of the complex scaffolds : the challenge is thus to make accessible complex molecules in a short number of steps to allow preparation of multigram quantities of the scaffold and build chemical libraries therefrom. We have completed our studies of a new class of cyclic dienes which could lead to scaffolds designed after natural products such as ottelione A, mesembrenone or lycorine. This class of activated and functionalized dienes should be of general interest to build complex nitrogen-containing molecules. They are readily accessible from the corresponding unsaturated lactames (Scheme 3).
Structurally and stereochemically complex structures are readily available in one or two steps from these dienes (Scheme 4). This project has now come to an end and a publication is in preparation.
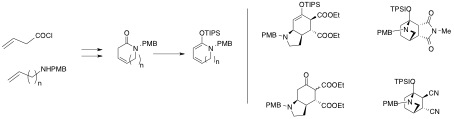
Scheme 3. Practical synthesis of the dienes -Scheme 4.Examples of complex structures available from dienes
3. The search for new ionic solvents and silicon-derived Lewis super acids
A few more applications of our silylated triflimides superacids and of the corresponding ionic solvents ( concentrated solutions of LiNTf2 in ethers, acetone, acetonitrile, ethyl acetate) have been found.
Selected publications
- Mendoza, O., Rossey, G., Ghosez,L. (2011). Brönstedt acid-catalyzed synthesis of diarylmethanes under non-genotoxic conditions. Tetrahedron Lett. 2011, ASAP
- Mendoza, O., Rossey, G., Ghosez, L . (2010). Trialkylsilyltriflimides as easily tunable organocatalysts for allylation and benzylation of silyl carbon nucleophiles with non-genotoxic reagents. Tetrahedron Lett. 2010, 51, 2571-2575 (cover)
- Zhu, W., Mena, M., Jnoff, E., Sun, N., Pasau, P., Ghosez, L. (2009). Multicomponent reactions for the synthesis of complex piperidine scaffolds. Angew. Chem. Int. Ed., 48, 5880-5883 (cover)
- Yang G., Ghosez, L. (2009). Synthesis of enantiopure ?-chlorocyclobutanones as scaffolds for the diverted total synthesis of serine protease inhibitors. Eur. J. Org. Chem., 111738-1748 (special issue, invited paper)
- Commandeur,M., Commandeur, C., De Paolis, M., Edmunds, A.J., Maienfisch , P., Ghosez, L. (2009). Studies related to the total synthesis of the sesquiterpene core of the pyrrolobenzoxazine natural product CJ-12662. Tetrahedron lett., 50, 3359-3362 (special issue, invited paper)
- Srinivas, K., Kauffmann, B., Dolain, C., LĂ©ger, J.M. , Ghosez, L., Huc., I. (2008). Remote substituent effects and regioselective enhancement of electrophilic substitutions in helical aromatic oligoamides. J. Am. Chem. Soc, 130: 13210-13211.
- Champeil, E., Crean, C., Larraya, C., Pescitelli, G., Proni, G., Ghosez, L. (2008). Functionalization of [60] fullerene via organometallic reagents. Tetrahedron, 64: 10319-10330.
- Akhatou, A., Rahini, M. Cheboub, K., Ghosez, L., Hanquet, G. (2007). Acid-promoted enan-tioselective oxygen-atom transfer from N-alkyl binaphtyl-derived oxaziridines onto sulfides. Tetrahedron, 63, 8449-8462 (invited paper, Tetrahedron 50th anniversary issue).
- Depré, D.; Chen, L.-Y.; Ghosez, L (2003). A short multigram asymmetric synthesis of prostanoid scaffolds. Tetrahedron, 59, 6797-6812
Research team
- Pr. Léon GHOSEZ Visiting scientist (Prof. Emerite, Université de Bordeaux)
- Dr. Eduard BADARAU Postdoctoral fellow (Université de Bordeaux)
- Michael ELSOM Master student (Erasmus University of Aberdeen)
- Magdalena PLUCIENNIK Master student (Erasmus Polit. Wroclawska)
The team is part of the unit “Chimie et Biologie des Membranes et Nanoobjets” (CBMN), CNRS/Université de Bordeaux/ENITAB (UMR 5248)
|