
Dr. Derek McCusker
Charg├® de Recherche (CR1), CNRS
Website
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
Tel: +33(0)540003021
Bio
Derek McCusker studied Immunology at Glasgow University and focused on the role of the proteasome in immunity in Prof. John TrowsdaleŌĆÖs lab at Cancer Research UK during his thesis. During postdoctoral work with Dr Robert Arkowitz at the Laboratory of Molecular Biology in Cambridge he became interested in the control of cell growth. He then joined Prof Douglas KelloggŌĆÖs group at the University of California, Santa Cruz, where he investigated how cells coordinate cell growth and cell division, a key problem in cell biology. He was recruited by CNRS in September 2009 and joined IECB as a group leader. The group uses interdisciplinary approaches to study how cell growth is coordinated with progression through the cell cycle.
Keywords / Expertise / Techniques
cell growth, cell cycle, cell polarity, Rho GTPase, endocytosis, exocytosis, cyclin-dependent kinase, phosphorylation, cell biology, TIRF-microscopy, membrane dynamics, biochemistry, mathematical modeling.
Summary
Cells grow, duplicate their genome and divide via a series of events collectively termed the cell cycle. Coordination between the cell cycle machinery and proteins that regulate cell growth ensure the fidelity of cell division; however, the underlying mechanisms are unclear. Failure of these control mechanisms has been directly linked to tumour formation. The goal of the Cell Growth and Division Laboratory is to understand how cell growth is controlled and how growth is coordinated with cell cycle progression. We address these fundamental ├é├é├é├é├é┬Āquestions using cutting edge interdisciplinary approaches.
Activity report
1. Robust polarity establishment via an endocytosis-based cortical corralling mechanism.
The size and shape that cells adopt is governed in part by the rate and location of plasma membrane growth. This growth is largely driven by exocytic vesicle fusion, while endocytosis removes membrane. The relative rates of these two processes therefore contributes to cell size. Moreover, the spatial organiaztion of these pathways contributes to cell shape and function. In budding yeast, endocytic and exocytic vesicles localize to growth sites in the bud. Given the antagonistic relationship of these processes, this arrangement could be incompatible with the membrane efflux required for polarized growth of the bud. Our previous work demonstrated that endocytic vesicles form a ring around exocytic sites at the cortex. To address whether this might facilitate the formation of a stable polarity axis that contributes to polarized growth, we studied the dynamics of endo- and exocytic vesicles using high resolution in vivo imaging and in silico mathematical modeling (Figure 1).
We made the following observations that were published in the Journal of Cell Biology:
- Robust polarity establishment involves dynamic changes in exocytic and endocytic trafficking systems (Figure 1).
- Robust polarity establishment involves the generation of a specific endocytic signature.
- Endocytic cortical corralling is required for robust polarity establishment.
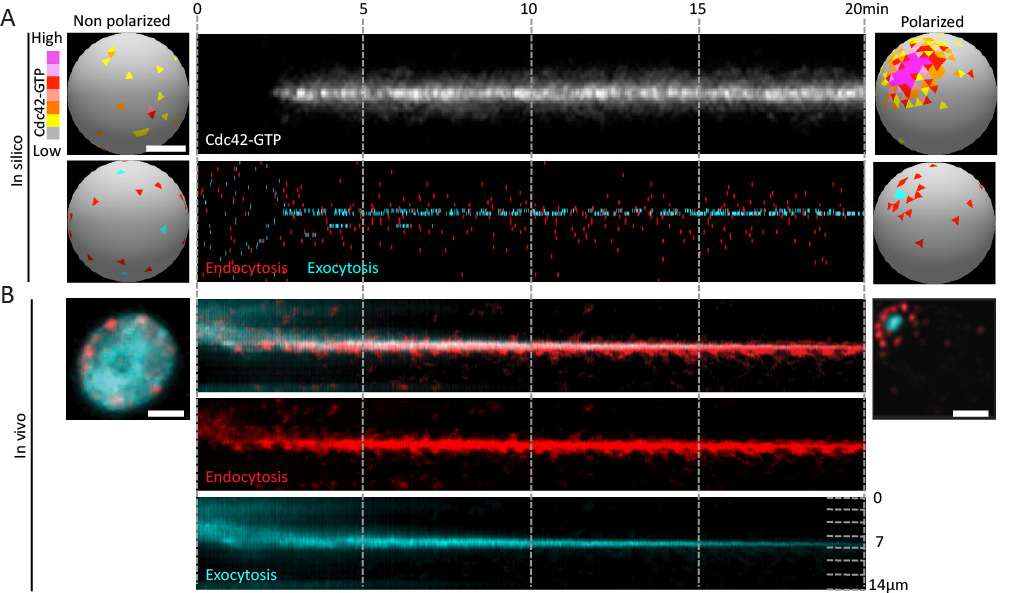
Figure 1. Robust polarity establishment involves dynamic changes in exocytic and endocytic trafficking systems. (A) Transition of an "in silico" cell from a non-polarized (left) to a polarized state. Membrane-bound active Cdc42 depolarized over the plasma membrane (top left) polarizes to a unique cluster of active Cdc42 over time (top right). The Cdc42 kymograph (upper panel) shows active Cdc42 during polarization. The kymograph in the lower panel shows individual endocytic and exocytic events over time (x-axis) along the cortex (y-axis). A tight pole of exocytosis develops (cyan) overlapping with the active Cdc42, and is corralled by a ring of endocytosis (red, bottom right). (B) Random endocytic and exocytic distributions in vivo in a non-polarized cell (left) change to an organized ŌĆśbullŌĆÖs-eye-patternŌĆÖ in a polarized cell (right) with a tight exocytic zone surrounded by endocytic vesicles.
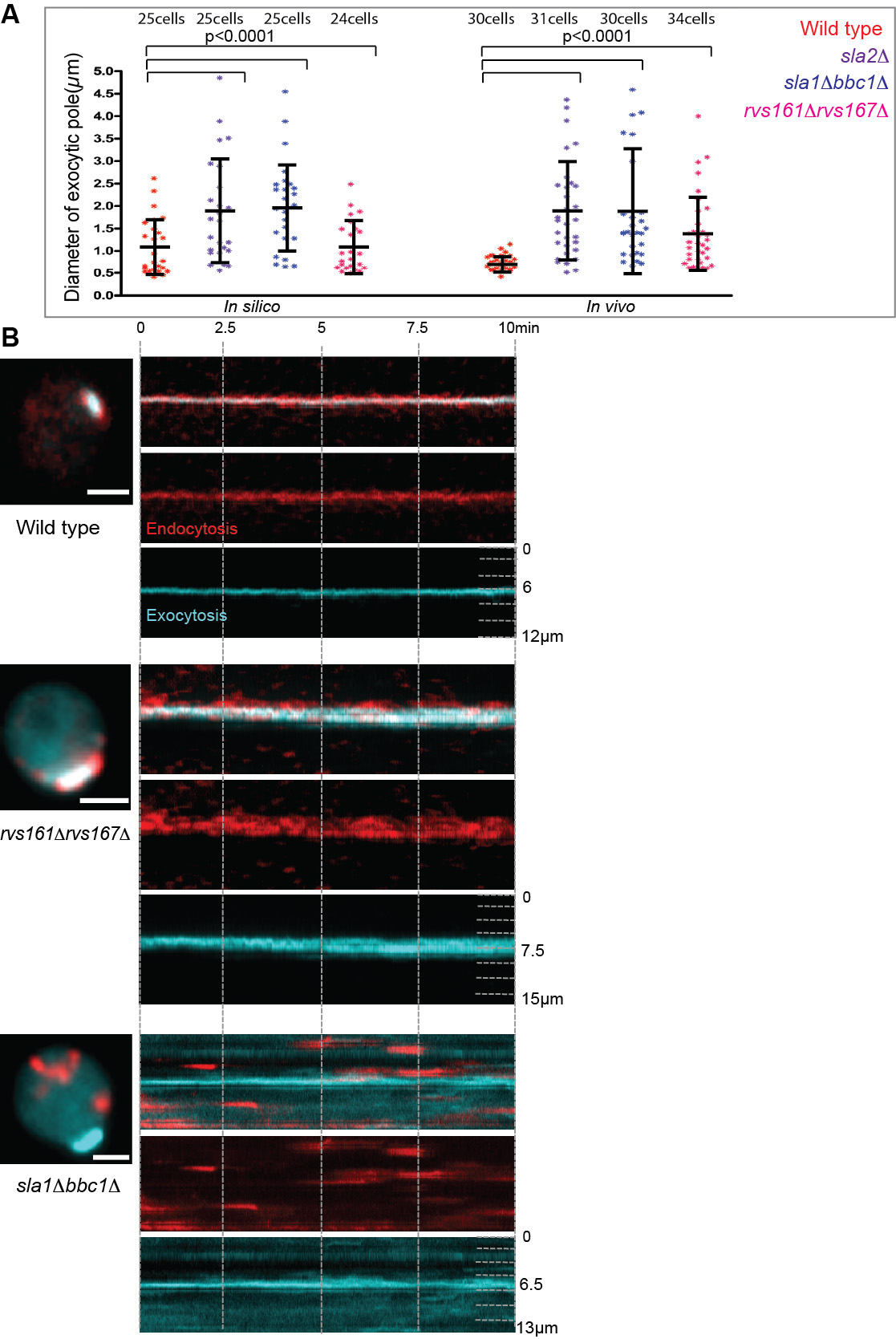
Figure 2. Endocytic cortical corralling is required for focused exocytic pole formation. The exocytic and endocytic vesicles are marked by GFP-Sec4 (cyan) and Abp1-RFP (red), respectively. (A) In silico and in vivo analyses of the distributions of exocytic cluster size for polarized wild type cells (red) compared to endocytic mutants such as sla2╬ö (purple), sla1╬öbbc1╬ö (blue) and rvs167╬örvs161╬ö cells (pink). An unpaired two-tailed test with WelchŌĆÖs correction showed significantly different mean values between the polarized wild type and mutant strains, except for in silico rvs167╬örvs161╬ö. (B) Kymograph of polarized wild type cells in vivo (upper panel) display focused exocytic pole (cyan) during polarization corralled by endocytic vesicles (red). Endocytic mutants defective in corralling such as sla1╬öbbc1╬ö (middle panel) and rvs167╬örvs161╬ö (lower panel) display depolarized endocytic vesicles (red) and wider exocytic poles (cyan).
2. A quantitative imaging-based screen reveals the exocyst vesicle tethering complex as a network hub connecting endo- and exocytosis.
Having identified a requirement for the spatial organization of endocytic sites around a developing exocytic pole in order to establish and maintain a robust polarity axis, we performed an imaging-based mutant screen to identify proteins required for this striking organization. Surprisingly little is known about the mechanisms linking these two essential trafficking pathways, despite intensive study of each individual pathway during the past 30 years. Developing methods in yeast cells that enable the tracking of vesicle dynamics, our studies identified many new players involved in the organization of endo- and exocytic trafficking domains.
In brief:
- We performed a candidate screen in over 400 deletions or temperature sensitive mutants and identified 120 mutants in which either endocytosis, exocytosis or both pathways are depolarized (Figure 3A).
- A functional interaction map of components affecting the spatial organization of endo- and exocytic sites revealed the exocyst vesicle-tethering complex as a hub linking the two processes.
- Exocyst mutants display a striking phenotype in which the organization of endo- and exocytic sites seen become interspersed.
- We introduce the use of super-resolution vesicle imaging and high density tracking to provide a novel quantitative view of vesicle dynamics in live cells (Figure 3B).
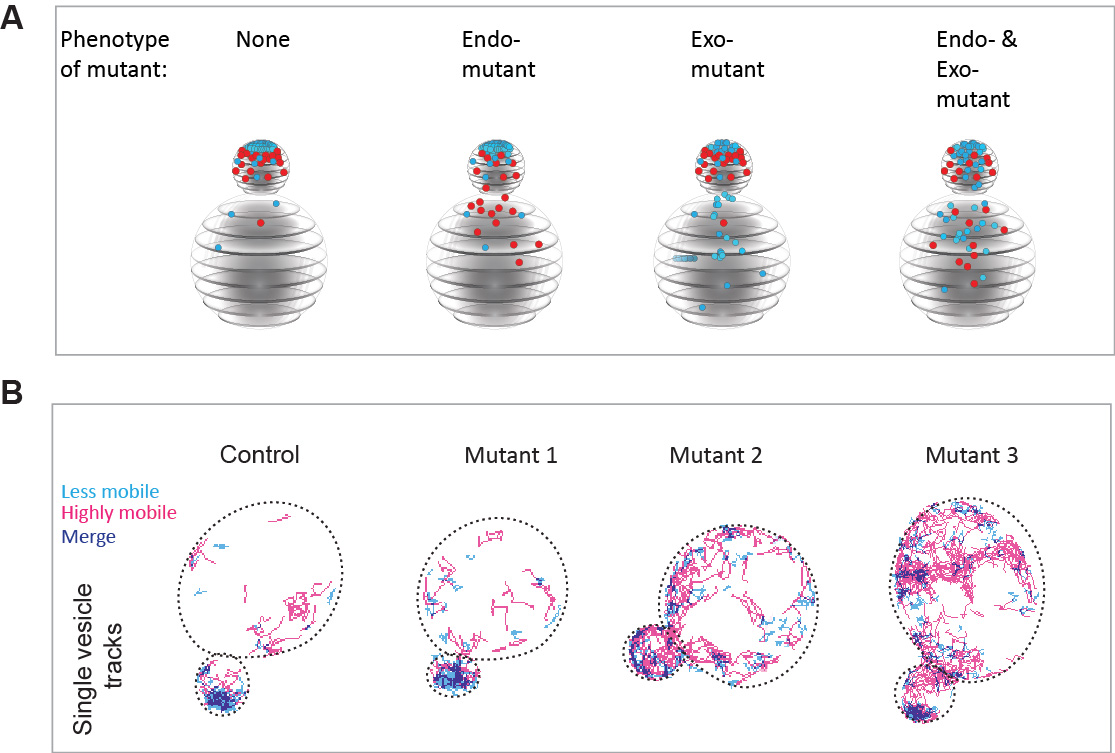
Figure 3. An imaging-based screening approach to identify proteins required for the spatial organization of endo- and exocytic trafficking domains. A Schematic representation of the mutant phenotypes identified by our screen. Endocytic vesicles are sown in red, while exocytic vesicles are shown in cyan. B Super-resolution imaging and very high-density tracking to monitor exocytic vesicle dynamics in the mutants identified by the screen. Using this approach, thousands of vesicle movements can be monitored in each cell.
Selected publications
- Jose M, Tollis S, Nair D, Mitteau R, Massoni-Laporte A, Velours C, Sibarita JB and McCusker D. A quantitative imaging-based screen reveals the exocyst complex as a network hub linking endo- and exocytosis. Molecular Biology of the Cell. 2015. 26: 2519-2534.
- Derive N, Landmann C, Montembault E, Claverie MC, Pierre-Elies P, Goutte-Gattat D, Founounou N, McCusker D, Royou A. Bub3-associated BubR1 sequesters Fizzy/Cdc20 at DNA breaks and promotes the correct segregation of broken chromosomes. The Journal of Cell Biology. 2015. 211: 517-32.
- Jose M, Tollis S, Nair D, Sibarita JB and McCusker D. Robust polarity establishment occurs via an endocytosis-based cortical corralling mechanism. The Journal of Cell Biology. 2013. 200: 407-418.
- McCusker D and Kellogg D. Membrane growth during the cell cycle: Unsolved mysteries and recent progress. Current Opinion in Cell Biology. 2012. 24: 845-851.
- Kellogg, D.* Royou, A. and McCusker, D.* Cdk1-dependent control of membrane trafficking dynamics. Molecular Biology of the Cell. 2012. July 5. 3336-3347. (* Shared senior co-authorship).
Research team
- Dr. Derek MCCUSKER Team leader
- Laure BATAILLE Research Engineer (CNRS)
- Aur├®lie MASSONI-LAPORTE Assistant Engineer (CNRS)
- Julien MECA PhD Student (Universit├® de Bordeaux)
- Dr. P├®ter RAPALI Postdoctoral Fellow (CNRS)
- Dr. Elodie SARTOREL Postdoctoral Fellow (CNRS)
- Dr. Caner UNLU Postdoctoral Fellow (CNRS)
This team is part of the ŌĆ£Institut de Biochimie et G├®n├®tique CellulaireŌĆØ (IBGC), CNRS/Universit├® Bordeaux (UMR5095)
|