
Â
Dr. Elisabeth GĂ©not
Directeur de recherche (DR2),
INSERM
Website
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
Tel: +33(0)540003056
Â
Bio
Elisabeth GĂ©not, trained in both biology and biochemistry at the University Pierre & Marie Curie in Paris, got her PhD in 1988 at the Curie Institute (Paris). Starting her career in immunology, she worked on the regulation of B lymphocyte expansion during the immune response and the molecular mechanisms underlying hairy cell leukemia oncogenicity. She trained in signal transduction at the University of Washington in Seattle (USA) under the guidance of Ed. Clark and Ed. Krebs and thereafter focused her work on intracellular signalling involving the RhoGTPase family of proteins at the LRI (London, UK). She started her own group at Imperial College in 1997, arrived at the University of Bordeaux in 2000 and joined IECB in 2002. Her current research focuses on endothelial cell biology in health and diseases.
Â
Keywords / Expertise / Techniques
GTPase, endothelial cells, adhesion, survival, cytoskeleton, podosomes, cell dynamics, Video-/confocal microscopy, transient and stable transfection, protein kinase activity assays, selective precipitation and in situ examination of active GTPases
Â
Summary
Transforming growth factor-Ăź plays an important role in the development and maintenance of homeostasis of the vascular systems by regulating functions of endothelial cells and smooth muscle cells. Analysing the effects of TGFĂź on cytoskeleton organisation led us to discover actin-rich structures named podosomes in aortic endothelial cells. Ongoing projects aim at demonstrating the existence of podosomes in vivo and determine their role in endothelial cell (patho)physiology. In vitro work aims at a full characterization of the molecular composition of podosomes and elucidation of the molecular mechanisms involved in their assembly and disassembly in both microvascular and macrovascular endothelial cells.
Â
Activity report
Our aim is to understand some of the mechanisms by which endothelial cells contribute to the pathophysiology of vascular diseases. We are studying how environmental cues impact on endothelial cells and translate into functional alterations focusing on changes in ECM composition/rigidity and cytokine contexts. TGFĂź plays a key role in cancer, fibrosis and inflammatory processes and endothelial cells represent a major target of its action. We focus our analysis on endothelial cell's cytoskeleton remodeling and differentiation in response to TGFĂź and accumulation of pathological matrix. Our studies aim at a better understanding of the signaling cascades underlying endothelial cell behaviour in human diseases such as tumoral angiogenesis and metastasis, inflammation or atherosclerosis, with the long term goal of manipulating these cascades for therapeutical intervention.
Our work has established that TGFĂź causes the repolymerisation of actin into punctate structures named podosomes. A podosome is made of a columnar actin-rich core standing perpendicular to the plane of the ventral plasma membrane and embedded in a ring structure of integrins and integrin-associated proteins. Other components include signalling molecules such as tyrosine-kinases, GTPases and effectors proteins as in focal adhesion. However, unlike focal adhesions, gelsolin, dynamin, cortactin and WASp/N-WASp are also detected. Another peculiarity of podosomes is that they are enriched in matrix metalloproteases, bestowing them with the capacity to degrade the ECM. Podosomes are found in a restricted number of cell types (macrophages, immature dendritic cells and osteoclasts) where they seem to be involved in adhesion and invasion. These cells share in common the ability to cross anatomical boundaries.
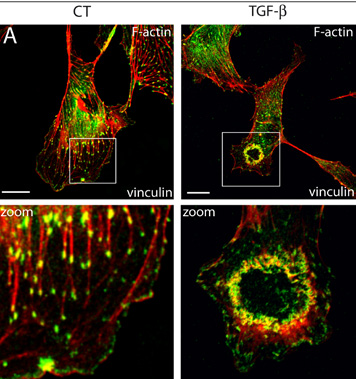
Figure 1. Endothelial podosome rosettes in cultured endothelial cells.
(A) Representative immunoconfocal images of F-actin (red) and vinculin (green) organisation in control (left panel) and TGFß-treated (right panel) cultured aortic endothelial cells. In untreated cells, vinculin is localised at the tips of stress fibers. TGFß-treated cells exhibit ring-like structures (podosome rosettes, right panel), where vinculin surrounds F-actin cores (tbottom panel: higher magnification of the boxed areasl). Bar, 10 µm
We have undertaken an extensive characterization of podosomes in different types of endothelial cells. These analyses have brought to light novel components not described previously, which could be involved in specific functions of endothelial cells. We are presently exploring the contribution of these molecules to podosome formation and functioning.
The question arises whether podosomes are also formed in physiological contexts. We therefore set up an “en face” viewing system to visualise the endothelium in its native environment. This system enabled us to visualise the cytoskeleton of endothelial cells in murine aortic vessel segments and establish that the normal endothelium is devoid of podosomes. However, upon exposure to physiological concentrations of TGFß, the formation of podosome rosettes was induced. The detection of podosomes in living tissues opens the way to investigate in which cellular process podosome forming cells are engaged.
Â
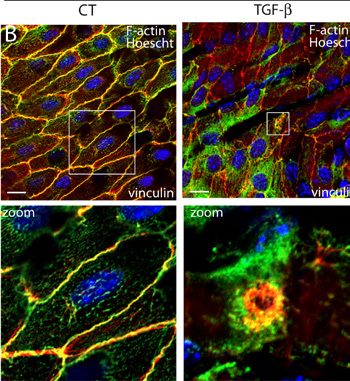
Figure 2: Endothelial podosome rosettes in the endothelium of an aortic vessel segment
(B) Aortic vessel segments were prepared for immunofluorescence, labelled with phalloidin (red), vinculin (green) and Hoechst (blue) and mounted en face for confocal observation. Viewed face on, vinculin/F-actin staining of the endothelium delineates individual cells in control condition (left panel). TGFĂź-treated aortic vessel segments (right panel) show the relocalisation of vinculin at the podosome rosette (bottom panel: higher magnification of the boxed areas). Bar, 10 ?m. Comparison of (A) and (B) reveals that podosome rosettes formed in their native environment are more compact than those assembled in the tissue culture conditions.
Â
We have now provided evidence for the existence of podosomes in living endothelia ex vivo in aortic endothelial cells obtained from inflamed endothelia, potentially under pro-angiogenic conditions, suggesting that podosomes are associated with a pathological state. We are particularly interested in the role of podosomes in vascular disorders involving hyperactivation of TGFĂź signalling pathways such as Marfan syndrome or those involving defective TGFĂź signalling such as Hereditary Hemorrhagic Teleangiectasia (HHT).
Â
Selected publications
-
Le Roux-Goglin E., Varon C., Spuul P., Asencio C., MĂ©graud F., GĂ©not E. (2012) Helicobacter infection induces podosome assembly in primary hepatocytes in vitro. Eur J Cell Biol. 91(3) p 161-170
-
Saltel F., Daubon T., Juin A., Ganuza I.E., Veillat V., GĂ©not E. (2011) Invadosomes: intriguing structures with promise., Eur J Cell Biol. Feb-Mar;90(2-3):100-7. Review.
-
Daubon T., Buccione R., GĂ©not E. (2011). The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in TGFb-stimulated aortic endothelial cells. Mol. Cell. Biol.,31(22): 4430-41
-
Quideau S., Douat-Casassus C., Delannoy LĂłpez D.M., Di Primo C., Chassaing S., Jacquet R., Saltel F., GĂ©not E. (2011) Binding of filamentous actin and winding into fibrillar aggregates by the polyphenolic C-glucosidic ellagitannin vescalagin. Angew Chem Int Ed Engl. 23;50(22):5099-104.
-
Rottiers P., Saltel F., Daubon T., Chaigne-Delalande B., Tridon V., Billottet C., Reuzeau E., and Genot E. (2009). TGFbeta-induced endothelial podo-somes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci, 122(Pt 23):4311-8.
-
Varon C., Tatin F., Moreau V., Van Obberghen-Schilling E., Fernandez-Sauze S., Reuzeau E., Kramer I., Genot E. (2006). Transforming Growth Factor {beta} Induces Rosettes of Podosomes in Primary Aortic Endothelial Cells. Mol Cell Biol., 26(9):3582-94.
-
Moreau V., Tatin F., Varon C., GĂ©not E. (2003). Actin can reorganise in podosomes in porcine aortic endothelial cells, a process controled by Cdc42 and RhoA. Mol. Cell. Biol., 23: 6809-6822.
-
Signal Transduction. Gomperts B.D., Kramer I.M., Tatham P.E.R., authors (2009). 2nd edition. Academic Press/Elsevier (ISBN-10:0-12-369441-8), 576 pages. Foreign editions: China, Japan, Korea
Â
Research team
- Dr. Elisabeth GÉNOT Team leader
- Dr. IJsbrand KRAMER Professor (PR1 Univ. Bordeaux 1)
- Edith REUZEAU Technician (TCE Univ. Bordeaux 1)
- Dr. Pirjo SPUUL Visiting Scientist (Finish fund)
- Dr. Thomas DAUBON Post-doc (ARC)
- Dr. VĂ©ronique VEILLAT Post-doc (ANR)
- Dr. Anne LECLERCQ Post-doc (FRM)
- Isabel EGANA PhD student (ITN-FP7-T3net)
- Filipa CURADO PhD student (ITN-FP7-T3net)
This team is part of the unit “Fibrose Hépatique et Cancer du Foie”, Université Bordeaux Segalen/INSERM (U1053)
|