| A first step towards the programmed assembly of water soluble peptidomimetic helices |
|
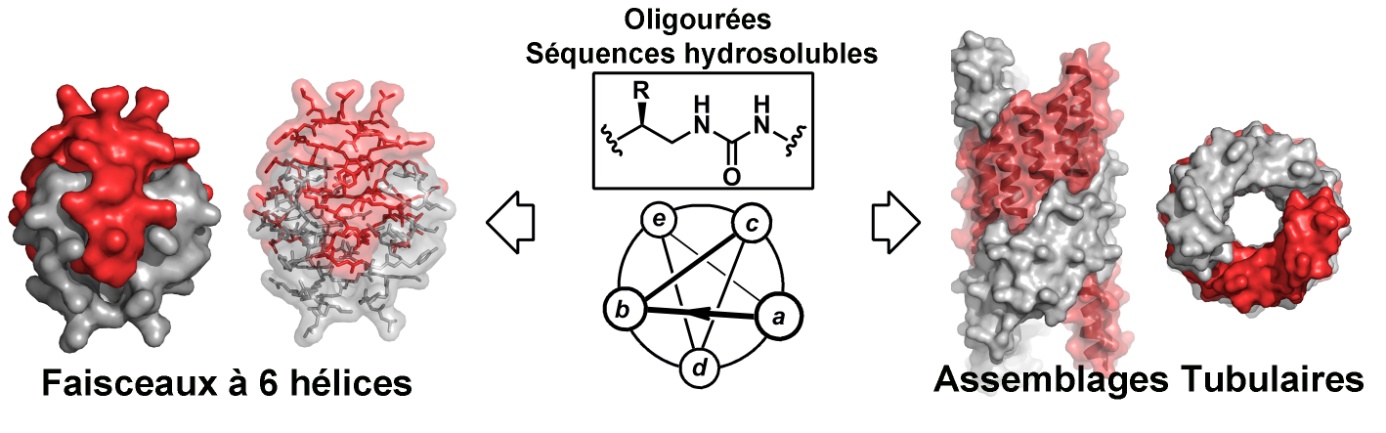
IECB teams have developed biomimetic helices with the ability to self-assemble into homogeneous protein like architectures in aqueous conditions. These results have just been released in the journal Nature Chemistry.
Proteins are polypeptide chains whose shapes and functions are intrinsically related to their primary structure (sequence of amino acid residues). The twenty residues available in living organisms to write these sequences encode the necessary information for the protein to fold into basic structural elements (helices, sheets, elbows) and their further assembly into a given tertiary and quaternary structure. Inspired by such polypeptide architectures, the team of Dr Gilles Guichard (CBMN, UMR 5248), in collaboration with those of Dr Olivier lambert (CBMN), Dr Valérie Gabelica (ARNA), and Dr Cameron Mackereth (ARNA) have successfully transposed folding and assembly processes to artificial (non-peptide) molecular chains to create nanostructures with unusual shapes (hexameric bundles and channels of various diameters - see Figure) in aqueous conditions. Unprecedented architectures obtained by spontaneous assembly of artificial water soluble oligourea helices. The researchers have used short oligomeric strands with urea units and proteinogenic side chains that can fold into well-defined helical structures. The sequences of these oligomers were designed to contain a certain proportion of residues with polar (hydrophilic) and nonpolar (hydrophobic) side chains which depending on their arrangement at the surface of the helix determine how the helices self-assemble in water. Similar to soluble proteins, the hydrophobic effect (which leads to the burial of nonpolar side chains, polar chains being exposed at the surface) is predominant. Sequence diversity enabled by automated synthesis of oligoureas suggests that the approach could be used to generate a much broader range of architectures of different stoichiometries and shapes. This work could contribute to the development of new functional nanomaterials, such as catalysts, biosensors or delivery systems. Reference Gavin W. Collie, Karolina Pulka-Ziach, Caterina M. Lombardo, Juliette Fremaux Frederic Rosu, Marion Decossas, Laura Mauran, Olivier Lambert, Valerie Gabelica, Cameron D. Mackereth & Gilles Guichard “Shaping quaternary assemblies of water-soluble non-helical peptide sequence foldamers by manipulation” Nature Chemistry – electronic release on September 28th, 2015 |
2, Rue Robert Escarpit - 33607 PESSAC - France
Tel. : +33 (5) 40 00 30 38 - Fax. : +33 (5) 40 00 30 68


 A first step towards the programmed assembly of water soluble peptidomimetic helices
A first step towards the programmed assembly of water soluble peptidomimetic helices